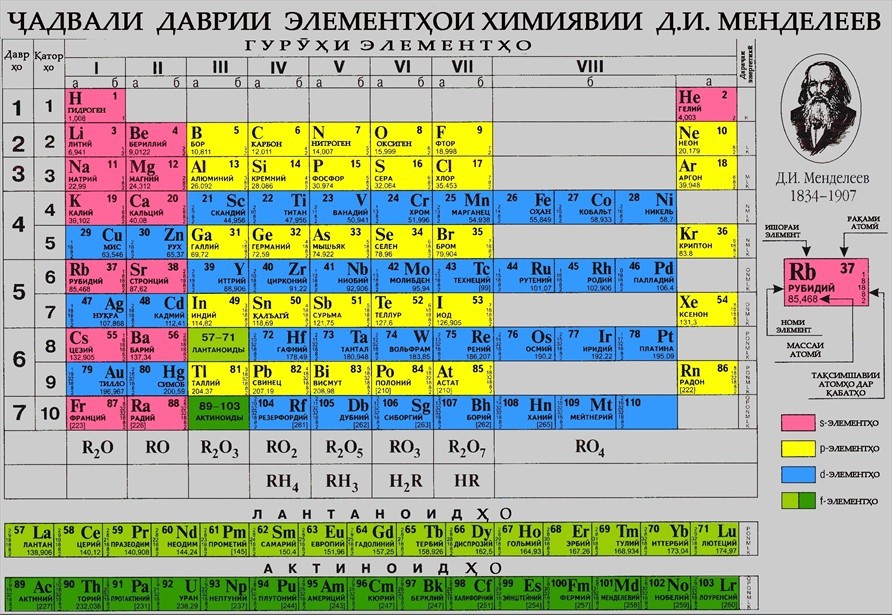
D. I. MENDELEEV'S TABLE - GUIDE TO CHEMISTRY
This year Mendeleev's table or periodic system of chemical elements is 155 years old. This table was discovered by the great Russian scientist D.I. Mendeleev at the age of 35, when only 63 elements were known. The greatness of the scientist was that he took the charge of the nucleus as the basic concept for the atom and changed the place of some elements in the periodic system. For example, element number 18 argon Ar = 40 to element number 19 potassium Ar = 39, cobalt to nickel, iodine to tellurium, etc. He also changed the atomic mass of beryllium, which was 13.5 at the time to 9: Li(7) +Na(11)/2 = 18/2 = 9.
Another greatness of the scientist was that he took into account the properties of the elements, predicted the existence of undiscovered elements, and left empty "rooms" in the Mendeleev table for them. Previously undiscovered chemical elements included scandium (Sc), gallium (Ga), and germanium (Ge). The predicted elements were discovered during Mendeleev's lifetime, and his predictions of their properties were confirmed with remarkable accuracy. The most important laws of nature - the law of transition of quantity into quality, the law of negation and negation and the law of unity and struggle of opposites - are connected with the periodic law and Mendeleev's table of chemical elements. That is, it shows that, without knowing chemistry, it is impossible to get detailed information about the basic laws of nature that surrounds us.
This year the world's chemists celebrate the 190th anniversary of the birth of the great Russian scientist Dmitry Ivanovich Mendeleev. D. I. Mendeleev was born on January 27 (February 8, 1834) in the city of Tobolsk (Russia) in the family of the director of the local gymnasium. He was the youngest child in the family (17th child). He received his elementary education at the local gymnasium. After graduating from the gymnasium, he entered the St. Petersburg Pedagogical Institute and graduated in 1857 with a gold medal. In his doctoral dissertation "On the bonding of alcohol and water and the understanding of solutions as associations" he discovered the theory of hydration, and in his outstanding work "Fundamentals of Chemistry" he explained all questions of inorganic chemistry on the basis of the periodic law.
Obidov Jamshed Mahmadnazarovich
c.ch.s., associate professor, department of chemistry
bioorganic and physicocolloidal
translated Ismoilov R.